When Chinese scientist He Jiankui first claimed that he was able to create the first genetically modified twin babies using the gene-editing technique CRISPR, the whole world was shocked.
“I believe this is going to help the families and their children,” He said in an interview with Associated Press, even saying that if the study ever caused harm, he would “feel the same pain as they do and it’s going to be [his] responsibility.”
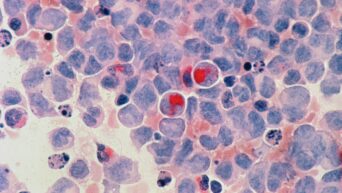
And now, the US Food and Drug Administration has approved the first CRISPR clinical trials outside of China, in partnership with Vertex Pharma. Although it’s still illegal to use genetically modified embryos in pregnancies in the United States as well in most other Western countries, the main purpose of these clinical trials will be to treat rare diseases, such as sickle cell disease and beta thalassemia.
The goal of the trials is to see if scientists will be able to modify cells that can be used in stem cell therapy. The first trial will be done on two patients who will each receive a dose of the CTX001, one of the CRISPR drugs. Scientists will be monitoring the results closely to see if the new method will have an effect on the patients.